dian
Members-
Posts
41 -
Joined
-
Last visited
Content Type
Profiles
Forums
Articles
Gallery
Downloads
Events
Everything posted by dian
-
Cast Steel, Cast Iron, Forging vs. Casting, and Understanding Grain
dian replied to Patrick Nowak's topic in Metallurgy
here some thoughts. grain flow: it depends if you look at some 100 ton slab a 50 ton part will be forged from, that might include 1/4" sized grains or a small low quality mild steel part that will be formed with a few blows (like the hook below) or a high quality part out of "good" alloy steel where forging is done to improve the microstucture (with quenching in between) or even some vim-var/esr melted, ultra fine grained steel. the first might really have elongated grains, the second elongated "inclusions", like fayalite stringers (or oxides of bi-films), the third will often have banding (segregations of constituens and elements) while the last will have a "clean", uniform and anisotropic structure. (thats when you would machine a crank from "billet".) typically you see the expression "grain flow" in older literature, where they used "dirty" steel, while lately "metal/material flow is used". so its really more about the orientations of segregated areas having better propertier in the longitudinal direction. grain size: grain refinement up to 1000x is possible with hot working (rolling, forging). usually grains get reduced considerably (2-30x) provided its done right. and its easy to do it wrong because success depends on so many variables. i have included the example of a simple hook, where grain reduction in the neck takes place on the last pass. so if you screw that up the part will probably brake. (notice that the grain is groving in the body with subsequent passes.) grain size is one of the rare ways to increase strenght and toughness simultaniously. But: that is (like always) with everything else being equal. example: you figure you want smaller grain size and reduce the forging temperature. good idea, however if this results in primary ferrite on grain boundaries (especially if its blocky) you have shot yourself in the foot. forging mild steel hook btw, if we talk about grains, we mean prior austenite grains. e.g in perlite colony size and interlamellar spacing are im portant. there will be a number of colonies in such a grain. sometimes a special heat treat is neccessary to make the prior austenite grains visible. hook.pdf https://core.ac.uk/reader/195109013 (i was talking about fig. 8.) -
Cast Steel, Cast Iron, Forging vs. Casting, and Understanding Grain
dian replied to Patrick Nowak's topic in Metallurgy
in a way your right, slip planes are denser areas in a crystal where dislocations move through. dislocations are created by plastic deformation, store energy and influence the processes taking place in the metal. e.g. a high dislocation density results in a lower recrystalization temperature (annealing), because less additional energy is needed for the same driving force and at the lower temperature there is less grain growth. or similarly, retained austenite converts to martensite at a lower temperature by strain induced twinning (tempering). during hot forming in the austenitic region the situation is different. strain induced recrystalization and grain growth happen simultatiously and the resulting effect on grain size depends on many factors, like amount of strain, strain rate, temperature gradient, alloy composition, phase transformation effects and others. usually the biggest concern is unequal strain distribution during forging. interestingly, the grain size does not depend on previous grain size (like with intercritical annealing), but is a function of temperature and strain. what happens during hand or small power hammer forging i have no idea. maybe somebody else knows. (search terms for further study: dynamic recrystalization (DRX), zener-hollomon, jonson-mehl-avrami-kolmogorov) some reading: https://iopscience.iop.org/article/10.1088/2053-1591/abd2f8/pdf -
edit: here is a short article that explains dual phase steel quite well. its older, but at least they seem to be using "plane-jane" 1018/1020 steel. they get considerably higher wear resistance compared to another 37 hrc steel. 0043-1648(95)06796-5.pdf does the link work? (it does on my computer.)
-
perhaps i have caused the confusion to a certain extend by being too lazy to use capital letters (old habit): "si 0.46%". the composition of the steel in question was: C 0.23%, Mn 0.54%, Si 0.46%, P 0.004%, S 0.0005%. intercritically annealed at: 800°c/2 hours resulted in 41 hrc, 1280 mpa, 72 joule, 64% martensite 825°c/3 hours resulted in 24 hrc, 935 mpa, 135 joule, 83% martensite. as to "claims", on the contrary, i was hoping maybe somebody on here would be able to confirm it or had further insights in to matter or would even go and reproduce it, as i dont have access to an electric oven at the moment. btw, martensite doesnt need much bulk carbon content to form. as soon as there is austenite, it can be transformed into martensite. depending on its carbon content martensite can be hard of softer. its possible to get large fractions of martensite in very low carbon steels (e.g. 0.05%). if that were not the case, a lot of modern steels (e.g. such as used for sheet metal for cars) woud not exist. if interested, look up "dual phase steels". so whats the end of the story? well, the controversy over hardening mild steel might just be based on different procedures used. if you austenize it at 950°c and quench it it wont work. intercritical annealing seems to yield results.
-
Cast Steel, Cast Iron, Forging vs. Casting, and Understanding Grain
dian replied to Patrick Nowak's topic in Metallurgy
sorry, i was not being clear, when i said: "interestingly high end cranks are made from "billet", which i assume is forged and heat treated (normalised?) bar.", i meant to say they are machined. so not forged. -
no idea what you are saying, frosty. and no idea who came up with 0.46% carbon. i thought we might have a discussion on the hardenability of mild steel. anyways, to answer my own question: as there is obviously not much carbon, a too large fraction of martensite means that its carbon content is low and it gets weaker. apparently there is an optimal proportion of ferrite/austenite you have to aim for before quenching. edit: jlp, there was no mention of the martesite type in the paper, but i wondered about this also. do you think there might be plate martensite at this carbon level at all?
-
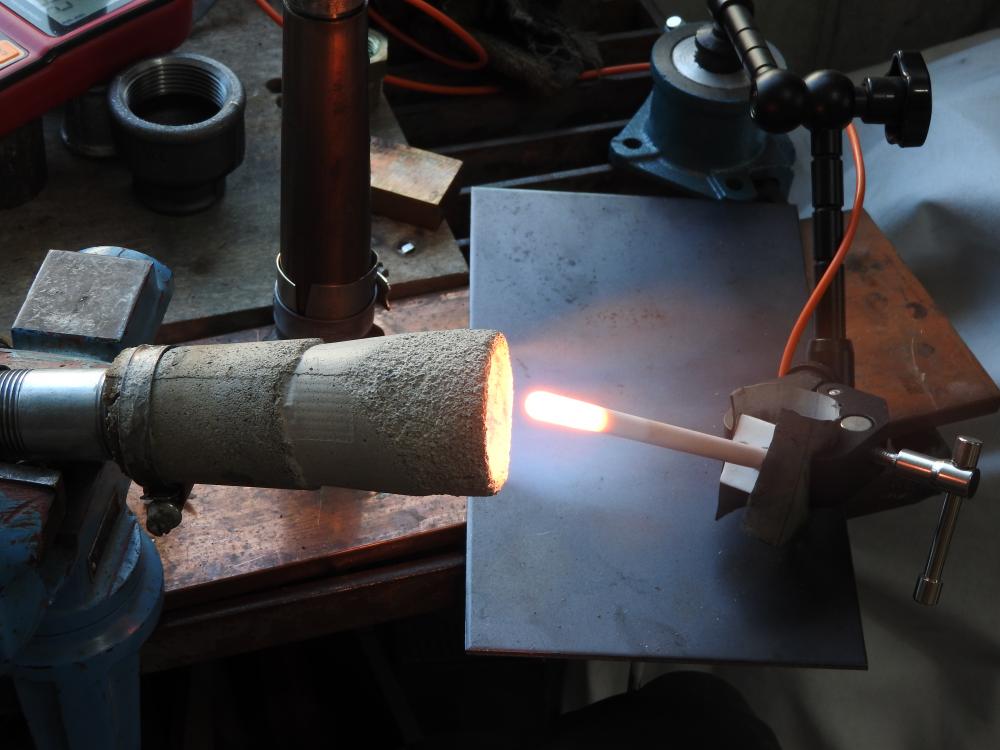
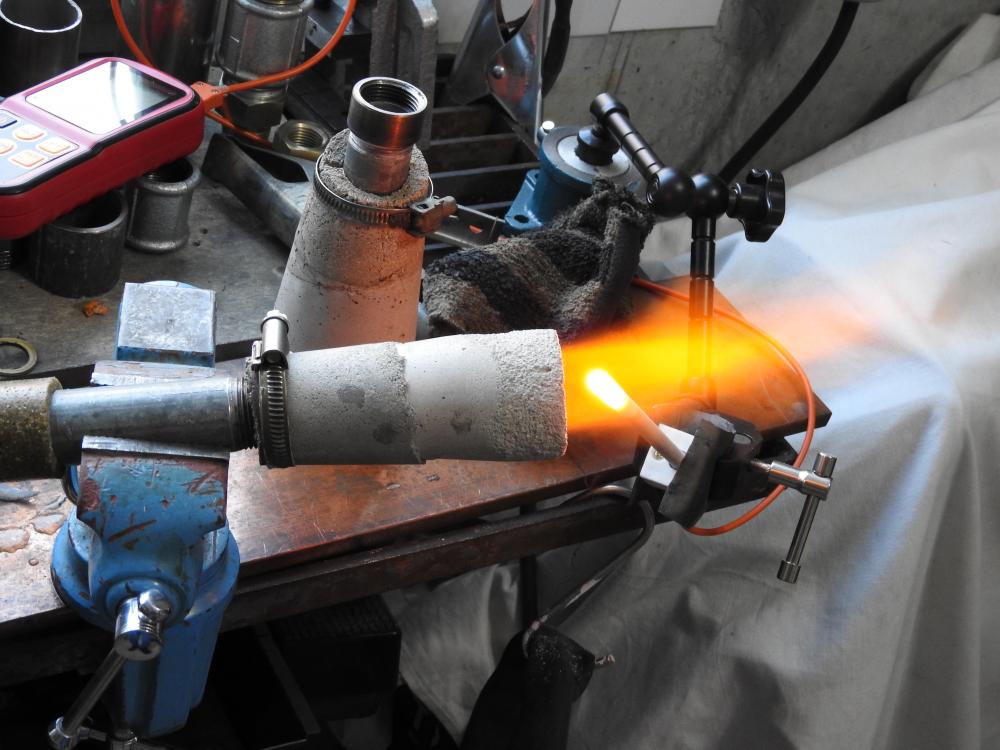
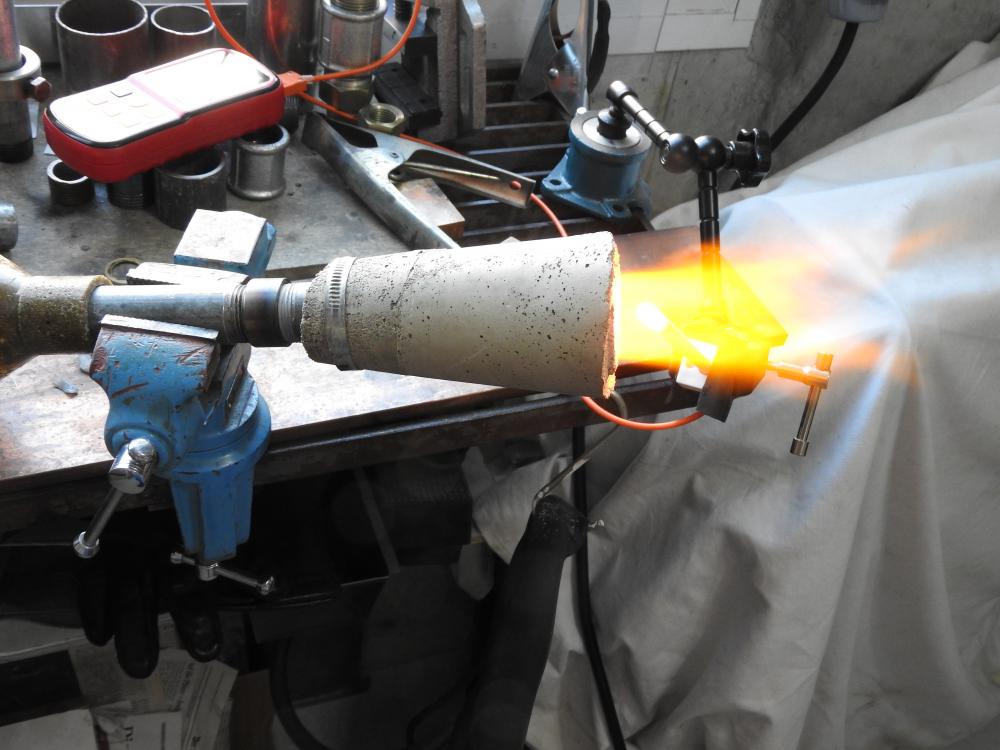
i got sidetracked by other stuff and got back to the project only now. i did some more experiments back then. the highest flame temp. i achieved was 2425°. i also tried to copy a design quite popular on youtube, without any "breakthrough". what kept me busy for a while is the fact, that the flame starts burning at the very start of the expansion tube. even when i really smoothed out the transition. then i made a burner for a smal table top oven, that has been working quite well (last picture, 12° included). i might be a bit obsessed with flame temperature. i quickly learned, that by putting an extension (tube) on a well burning flare you can raise the temp. a little, the gas gets uniformly hot. but i decided to use burners where the combustion fully takes place in the flare, no real flame commimg out. it might be true, that the gas will burn in the forge anyway, but this way there is at least no oxygen inside. well, in the end i found wind being quite a problem for flame stability and decided to go with a forced air burner.
-
do you know the answer? its "simple".
-
so apparently 1020 steel after some intercritical treatment and quench in ice water will have: uts 1280 mpa, 41 hrc, charpy 78 j. or: uts 935 mpa, 24 hrc, charpy 135 j. the structure will be ferrite + martensite and the secret is to find the right ratio. now, the steel used in that experiment had a little more si than standart 1020 (0.46%) and was unusually purified as to s + p. no idea if that is some "dual phase" alloy or what. but even ordinary 1020/1018 should respond to such a treatment. if i had an electric oven i would run and try it. so no need for tool steel anymore? something to contemplate for the interested: the steel got softer if the martensite fraction was large.
-
Cast Steel, Cast Iron, Forging vs. Casting, and Understanding Grain
dian replied to Patrick Nowak's topic in Metallurgy
imo the most important thing in any kind of metal working is to know exactly what you are dealing with. it starts with melting process (e.g. if vim/var you can assume a certain cleanliness), grain size, impurity level, heat treat (normalized, annealed, spheroidized, q+t) and exact composition (e.g. L2 too broad to be usefull). there is more. you have to find a supplier that is willing to give out as much info as possible. even an analysis on the optical microscope level doesnt tell much. -
4130 and such is nice if you need some springiness in a part (e.g. some flexture). you quench it and thats it.
-
Cast Steel, Cast Iron, Forging vs. Casting, and Understanding Grain
dian replied to Patrick Nowak's topic in Metallurgy
patric, if you forge a part, you would probably normalise it, austenize, quench and temper it. or you choose some sort of "intercritical" treatment to refine grain and carbide in a perlitic structure. (or, or ...). so how much of the "grain flow" you mention will survive heat treatment? to what extend and in which case? interestingly high end cranks are made from "billet", which i assume is forged and heat treated (normalised?) bar. (it might be worth mentioning, that ci categories are not "god given". there is a number of other types and an even larger number of intermediate types. e.g. usually you dont get pure lamellar graphite, there will be some globular fraction. the smaller it is the higher quality the gray iron.) roman -
cast iron or ductile cast iron?? how do you tell the difference ??
dian replied to sidesaddle queen's topic in Metallurgy
how to delete post? -
dont rely on it being L6. circular blades are made from a variety of "simpler" steels. over here 1.2235 is popular, often translated with l2, althoug L2 can really be anything. 1.2235:
-
passivation "strength" (in order of effectiveness): nitric, citric, phoshoric. of course depends on concentration too.
-
iv seen guys reach 2600°f (i believe with copressed air) and really wonder what makes the difference. i dont think it is a/f ratio, as i adjust this to highest temperature for each measurement. actually im doing this for a friend. he bought a two burner forge for $ 3000 (swiss prices, you know) and cant get to forging temperature with propane. of course as much depends on the forge as the flame, but if the flame is not hot enough you get nowhere, right?. it should be much easier to tune the burner in the forge im building once it runs hot it open air. im trying to get as high as possible, because the next project will be a melting furnace for cast iron (hopefully). i mean, if i get the flame hot enough i could even melt steel. concerning the lengh of the tube, are you measuring total lenght including the flare? i wonder how to translate this to my setup with the 4" long cone. using 3"of tube before the cone is an absolute minimum, it really only starts working with 4". thats 8"/id of 0.82" = 9.7.
-
its all very simple: 1. the couplers are there to be able to easily modify the length. the pipes almost touch and i dont see any detrimental effect. 2. i wanted the choke to be deep in the throat, so i can be moved out of the way and also to reduce the sensitivity. together with the position of the nozzle optimized before it has to be after the nozzle and i assumed the "ball-choke" woud flow more than a simple disc that would have to have a long stem anyway. 3. once i get the new forge built i can see what the burner does in there. however it will be much, much harder to tune it in the forge, e.g. the location of the probe has to be changed for every measurement. also because of the inertia if i were to measure forge (not flame) temperature. so far the experiments are encouraging yielding a 200°f difference to a "traditional" setup. 3. i was talking about injector stacks not carburators. so the interesting question is: where does the increase of 200°f come from? how can it be explained? then, and on the basis of above, where do i go from there? decrease the angle further, play with the lenght of the expansion cone, what else? (the blue flame is actually burning in the first third of the cones and what we see are the hot gasses colored by some traces of waterglass.) any ideas on further improvement of the flame temperatures will be appreciated.
-
i didnt know vinegar would remove zink. the conventional way is muriatic acid. and even then, if you have a part submerged in a sufficiantly large quantity of lets say 20% muriatic it will take a couple of hours, at least at room temperature.
-
one could also copy a carburetor or injection stack, but how they work with gas induction would have to be seen. anyway, the basis is the classic pipe-fitting-burner that produces a flame temperature of 2165/2206°f (45/60 psi). the position of the probe is optimised for each measurement and choke adjusted to max. temperature. as the flame tends to get hotter with time and there are some "inexplicable" fluctuations there is some scatter to the data and i took the highes achieved. my setup consists of an inexpensive instrument and probe, combined with a professional compensating cable (that cost me more than the rest, because i had to get it locally). therefore the absolute values are a bit questionable and i will be looking at the differences. everything with my obviously "magic-stubby-nozzle". (1) same with shorter pipe: 0/+62° (2) same setup with cat-liter intake (last picture): +15/+22: not much gain. this is not surprising, because all my burners can be leaned out until they go out in free air. but consider this: inspite of the minimal opening of the choke, once in my small (experimental) forge it can be completely opened up without the flame going out. thats why im trying to get more air in there. (3) not relevant for what im doing here, but just for the hell of it (compare with 2165/2206): - machined fitting: -27/-18 (dont mess with the fittings, they do exactly what the are "supposed" to do, for some reason.) - expansion pipe: -10/+94 (maybe im onto something here) - welding reduction: -14/-10 then the cast expansion pipes: (4) big (16° included, length 3.15"): +81/? (5) small (12°, same lenght): +182/+216 (4), (5) with cat-intake, all numbers referring to "2165/2206°f.
-
cant edit post. why is that? so the drop in flame temperature was 190°f. (had to look it up.)
-
propulsion gasses exit on the big end. i think my coke funnel has the shape you are talking about. weirdly enough they are the only ones among all the other plastic bottles, the problem being that i dont drink the stuff. the convex shape is in the throat and the rest is there to stabilize the air (and prevent wind messing with it, as i hope). it bugged me that there was not much difference in the position of my nozzle, so i figured it might need to go deeper into the throat, leading to the tapered variety (same size orifice). this one reaches into the steel pipe itself but results were the same, i ended up with the same position as before. what i found, however, was that now i could fully open the cardboad-choke, so the suction was less and i got a lower temperature as well (10000f less). as the nozzle is step drilled it occured to me, that it might not be flowing as much as the "stubby-nozzle". but going from 45 to 60 psi didnt make any difference. any thoughts on that?
-
trying to find best position of the nozzle by replacing the choke with a cardboard-choke. i figured the smaller the opening at a constant flame temperatue the more "suction" im getting. well, it turns out the nozzle can be almost anywhere, the difference being a few degrees. i then settled on what seemed slightly the best.
-
-
as the experiments looked promissing i started building the burner. i made the outside intake out of kitty-litter-epoxy. it runs on the slightest opening of the choke. the inside shape looks like a problem because the wall has to be very thin for the secondary air (siphon?) effect to work . im trying to find/think of something out of metal. the best so far is a thermo-bottle i have, but the throat is too big. does anybody have a good idea without me having to take up metal spinning? the shape, btw, with its parallel inlet and outlet walls is inspired by some rocket jets i came accross. i thought if i works well for propulsion why not as an intake?