-
Posts
664 -
Joined
-
Last visited
Content Type
Profiles
Forums
Articles
Gallery
Downloads
Events
Posts posted by DanielC
-
-
Heat treatment is similar to normal modern steels, though playing around with it can alter the appearance of the pattern.
I find that it works well in the high hardness ranges, but it starts very high. This particular flavor of steel gets harder than anything I've ever dealt with, and I play with steels like 1.2519, 1.2442, 125SC, Aogami1/2, Shirogami2, 26C3, W2, 1095, etc...I treat all of these steels in the 64-66rc range with thin edges. I make hocho style japanese knives and shape with stones and stone polish for kasumi finishes a lot. With that said this particular alloy has a higher top hardness. I don't have a rockwell tester at the moment as I've relied on Mathew Parkinsons hardness chisels over the years and really didn't need to know anything beyond 64-66rc, so I just haven't purchased one. However the steel reacts to my diamond plated as harder than anything over ever lain on them. I would estimate 68-70rc, which after talking to a few other wootz friends, this isn't unheard of at all. Not to mention the steel at this carbon concentration is around 25% iron carbide by volume, which cementite usually pegs around 71rc.
I try to dial it back to 64 (which takes a bit over 400F to get down to) for realistic hardnesses that I'm used to. Doesn't seem any more or less chippy for me, but I'm very careful with geometry/edge geometry so that always helps.
-
Thanks. This puck has been forged and made into 3 kitchen knives and a section of it is promised to one of my favorite Master Smiths for his own personal carry.
Im not finished with any of the knives. The previous pictures and even this were only after one quick dip in diluted nitric acid. They are due for a coffee dip next to make the darks darker.
-
-
My friend Mac McKinnon told me about that. Improper welding glasses or something. Needed at least shade 5?
-
It's possible but....
I'm going on a limb here don't take offense this is just observation...
There was a time period when some of the older smiths (older than me, prevalent smiths in the 80s and 90s) that thought using thermite was a good way to make alloyed steels. It can be, and it can get a lot hotter than conventional means..temps that can melt some high melting point pure metals like Tungsten. It was just a big thing among the mastersmith Rybar and others. Maybe those came from the thermite crowd.
Ferro alloys have lower melting temps than their respective pure alloys, and blend well with iron and steel. They are used today in steel production but seemingly not easy to get.
The magic is in the forging though. The forging is specific to what's in the Ingot as everything matters.
-
These are carbide formers. When the ingot solidifies, these trace elements precipitate out first and make their place in the Ingot very stable. Chemistry, melt temp, solidification rate, ingot preparation, and forging afterward is a consistent, very well orchestrated process. These will be gathering points for cementite later. The steel is worked at specific temps without failure every time. I used to grind into the bar and look at what structures I have going on under the scope to know when to begin forging at different temperatures, but it's now second nature.
Much like it became second nature to the smiths in India, Turkey or Syria. Except they were producing dozens and dozens of ingots at a time. They learned by repetition.
Edit: I have several kilos of FerroVanadium, FerroChromium, FerroNiobium, FerroTungsten, etc..
Other steels with these elements can be used in the mix as well. In the end, reducing ore isn't all that important for the final.product. That is just chemistry.
-
This is 1.6% C with traces of V and Cr.
This is basically the end goal for crucible steel endeavors. You can search back when I first joined and got into this in 2012 that my main driving force into any of this was Ric Furrer and his Nova special on crucible steel.
Now we circle back 10 years later and granted I've been successfully doing it for a few years now, it is in my 10th year of forge work that I've met some goals I made in the beginning, but may have even then exceeded my expectations.
Many crystalline patterns or stretched grain boundary cementite patterns out there, but very few really good lamellar water patterns out there, and hitting that goal has been met. Now I can just have fun with it. I've got many ingots ready to work into magic.
-
I don't remember the rules on quoting and all that, but thanks. It's a lamellar water pattern. Like the antiques.
-
-
I forged that puck out but it contained a void that stretched with the bar and didnt show up until I had a bar about a foot long.
I remelted it with other material and Manganese addition. The puck has recieved REM treatment and is ready to be forged out.
I'm working on a pile of sanmai at the moment though. The plan is to work the puck once I knock a group of sanmai out.
-
This is indeed a horizontal disc grinder. I made this one actually, modelled after the ine KMG makes.
1hp motor with a KBAC VFD for total speed control.
I basically plasma cut out of .250" 1018 plate a motor mount. Install motor, vfd and disc.
I spent around half the cost of the KMG model.
-
Here is another one with a core steelcsimilar to Aogami 1 called 1.2442.
This knife is a little different in that the wide bevel is coherent to the forged surface, and will be stone polished. These pictures show the multi-faceted bevel that is called Hamaguri in Japan. I take the edge to .001-.002" on a disc to 220, and then take it into my home where I will begin my process of stone polish. By the time I'm moving into natural stones from synthetics, the edge will be 0" and rather forming a burr.
Usually I sharpen a secondary bevel in but ive had some requests not to. This would be reserved for pretty much only the most serious stone polishers to be practical, as the act of polishing the entire wide bevel is also sharpening, much like sharpening single bevel knives from Japan, except you are forming a burr twice.
-
-
-
No, not at all I've had my sights on a few 15hp rolling mills, but until I get my big, big hammer set up, it isnt in the cards.
A modified mcdonald rolling mill will be fine for knife size billets after I hammer them down a bit.
-
Yea these took a bit more time than that. I am in the process of building a rolling mill, but until then, I just draw it carefully out under the power hammer.
-
-
Working on a big batch of sanmai. About 11 pounds worth of sanmai actually. Most of it is straight low carbon jacket steel sanmai, and a small portion was 410 SS jacket sanmai.
All of the billets contain a flavor of German tungsten carbon steels that resemble what's available to Japan from Hitachi.
I've had problems with SS sanmai in the past, but was able to pull through this time. Forged quite thin as well. All 3 knives have a spine thickness above the heel .105" thick or thinner (the small blade is like .089" above heel at spine.
Anyone who has forged SS sanmai, knows forging it thin is quite dangerous as the SS likes to be HOT or the billet wants to break apart.
Heat treated this batch along with some test swatches to check my HT of these difficult steels, with great success. Both the SS and core steel display silky tiny grain, even zoomed in. Core steels are 1.2519, 1.2442, and 26C3 ( not a tungsten steel)
More to come.
-
Thanks guys. She came out pretty clean

-
So my family draws names each year on who to focus a gift on, and I drew my brother, who of which I already felt I owed a knife to any way.
He was quite pleased.
I dont do straight monosteel often, but I had the month of December to get something together while working on everything else.
1.2002 steel (26C3), ironwood wa style handle. Simple poplar says. Ti pin. Thin stock, convex grind. RC64, lol. This steel is decently tough at this thickness and hardness.
-
An increase in carbon with the lack of most other carbides lends to edge stability. A 1.2 or 1.3% C edge can be slightly thinner at the same hardness as 1095, which is why these types of steels exist. White and blue paper and various german file steels rely on these slight metallurgical differences to squeeze out as much as you can from steel for high end cutting edges. It is also important to not that these steels were designed for this specific purpose, as opposed to being an alloy we use that was first developed with a different application in mind, like 52100 for example, which is arguably one of the best western steels used in cutlery.
As far as the cementation process, yes it can be done. It depends on time and temp. You may even have some neat banding in the end.
-
Yep, my spelling was off unfortunately. Ledeburite.
I have quite a variety of gray cast I've made on accident that formed in different structures depending on rate if cooling.
-
Perhaps similar. I am not sure the mechanical properties dendritic cementite are the same for graphite in needle form, but maybe. The two processes impart similar properties to the elasticity of the metal, so maybe.
With cast irons you have a very high concentration of cementite in the form of ludabrite, and if Si levels are high enough (1-3%), it doesnt allow carbon into solution and precipitates graphite needles. Depending on heating conditions these needles can be in primary cementite or not. The carbon content will also dictate this as well, as you go above 4% or roundabouts regardless of Si content.
I've always imagined graphite being pockets of porosity among the hard ceramic ludabrite, and then becoming stress risers during a cold bend, rendering a snap. I assumed this is why white cast iron is both forgeable and is more durable. These are my assumptions though as I dont dive too deeply into cast irons except during the occassions that I make them, see them in the scope and read about them in literature.
Also, this material is a little different than general steels. Imagine a sea of pearlite (or martensite if its hardened) with very hard ceramic waves (cementite).
This material gave swords the ability to be knife hard, and not snap when bent. It also gives a lot of edge retention and stability.
-
Gwen
I dont. I would consider it slow, but not the slowest I've ever heard. I cut back the juice for about 10min andnthen fully shut down, closing all entry points and letting it cool off in the furnace. A slow cool enhances cluster sheet spacing among the dendrites, and the final pattern is partly a result to this. Too slow and yes, pockets of CO2 form and could possibly be filled with precipitated graphite which will in turn wreck a pattern and create cold shut streamers containing graphite.
Tbh, that attempt was a little different than my usual and did not incorporate a means of burning any O2 in the steel as it was liquid. I tried using CaC as an oxidizer instead, but it seems to have failed me. I feel like this is a result of that, but I could be wrong.
A good way to check later on is to use my ruler for microscopy and measure the distance between aligned bands. If they are wider than 80 or so microns then it's probably a result of too slow of a cool. There is a fix to this however, and it is employed early on.
The ingot is quite large, and there are narrow crucibles available, however the more cycles ran and the more distance forged, the better things get.
Ohio
Thats a good documentary. I also use beer bottles. Preferably green. I had to stomach Heineken over the weekend for this last run.
I use commonly sourced graphite/clay crucibles. These are #3's, but I'm thinking about stepping up to #4s






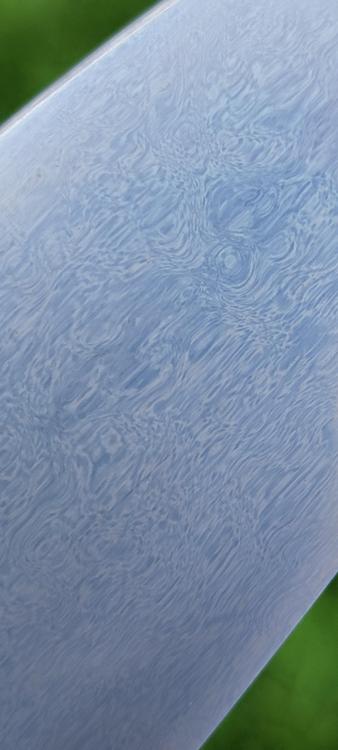

























First Crucible Steel Run, and Forging.
in Smelting, Melting, Foundry, and Casting
Posted
Coffee etching :]